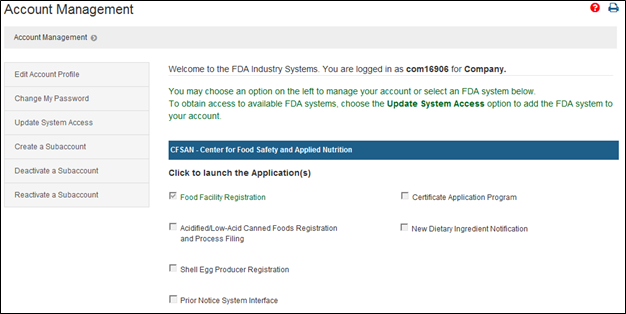
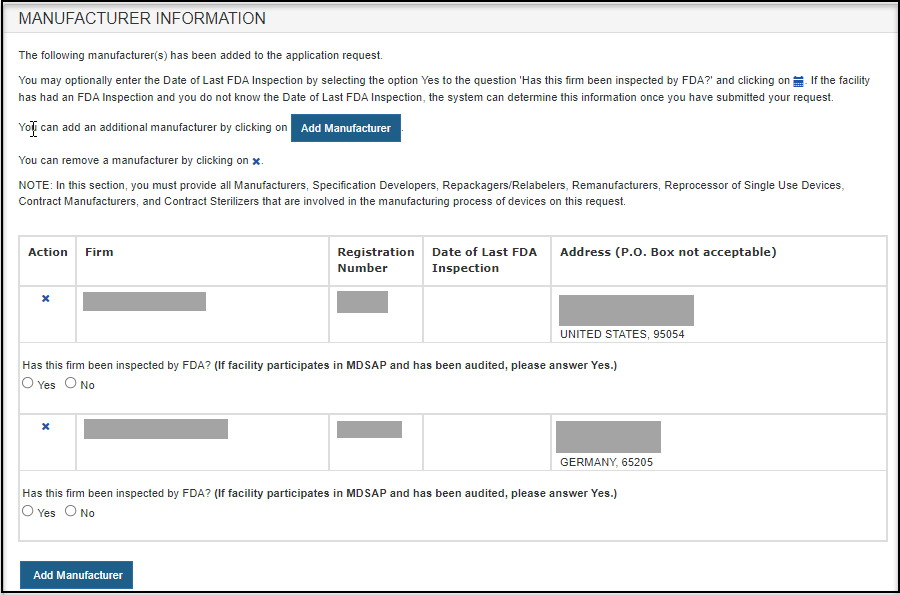
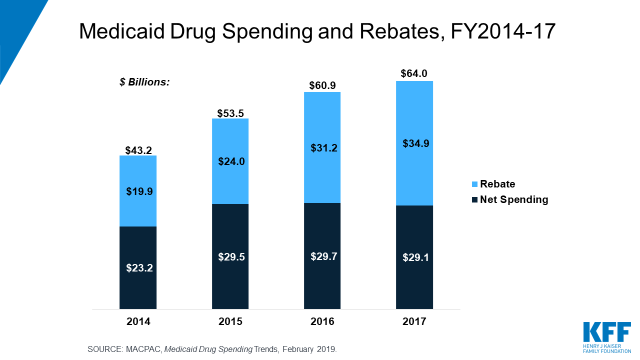
FDA Food Facility Registration is required under laws created by both the Bioterrorism Act of 2003 and Food Safety Modernization
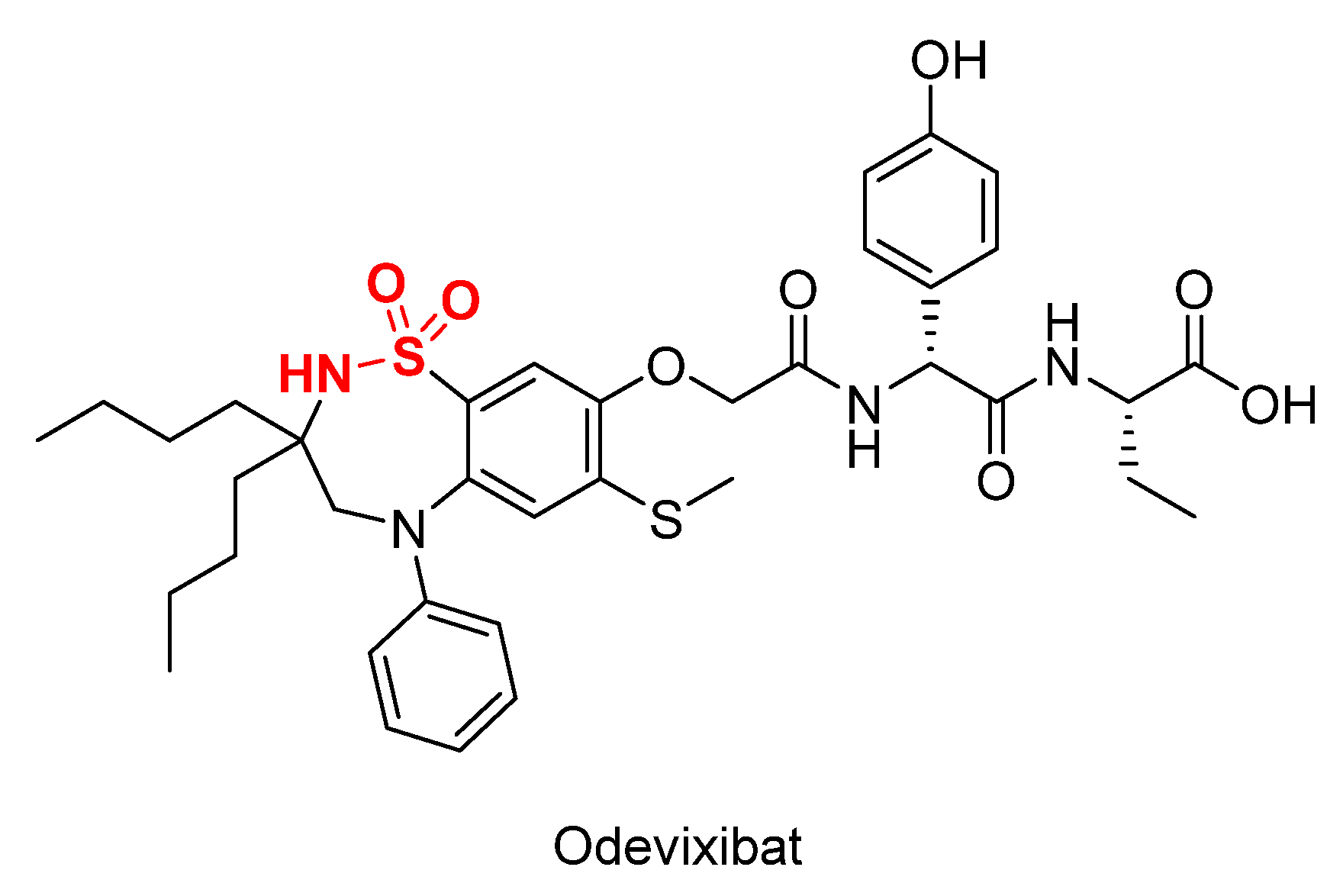
Pharmaceutics | Free Full-Text | FDA-Approved Small Molecules in 2022: Clinical Uses and Their Synthesis

Summary of Guidance for Minimizing the Impact of COVID-19 on Individual Persons, Communities, and Health Care Systems — United States, August 2022 | MMWR